CoP Containment
About
In ISPE D/A/CH, members are actively involved in regional working groups and the CoP’s (Communities of Practice). Through technical/topic-based workshops, discussions, and communication, best practice working papers and approaches are developed in the sensitive environment of patient safety. They serve as guidelines for the industry and the authorities. Members of ISPE D/A/CH participate in the ISPE Guides.
Introduction
Containment
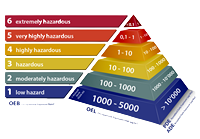
The topic of containment moves the pharmaceutical industry. Many newly introduced drugs are effective even in small doses – their ingredients are highly active. For patients, this development is an opportunity; for manufacturers, it is a challenge. Low occupational exposure limits and complex toxicological assessments require special measures for process safety in the laboratory and in production.
The new Containment Manual
Now available – the new edition of the successful Containment Handbook of the Working Group.
The German Containment Handbook in the 2nd edition is available as a book.
The manual covers risk assessment, containment life cycle, process requirements, technical systems, validation, cleaning and personnel. Each chapter of the handbook begins with a definition or introduction, concepts, processes, technologies are identified and described in the form of best practices or as illustrated examples. The new EMA guidelines, “Guideline on setting health based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities”, are also taken into account.


Lead CoP Containment
Dr. Martin Schöler
Fette Compacting GmbH

Co-lead
Michael Maintok
Glatt GmbH

Secretary
Jessica Redmer
Aicuris AG
Steering Committee
Hristina Freitag,
Vetter Pharma-Fertigungs GmbH & Co. KG
Dirk Collins,
WALDNER Process & Automation Solutions (PAS)
Oliver Gottlieb,
NNE (Dänemark)
Dr. Patrick Sproll,
Lonza AG
Uwe Hahmann,
HET Filter GmbH
Kolja Klein,
Pharmaplan AG
Daniela Kovats,
Fareva S.A.
Dirk Daegele,
Vetter Pharma-Fertigung GmbH & Co. KG
Dr. Andreas Schreiner,
Novartis Phama AG
Bernhard Steidle,
R-Pharm Germany GmbH
Christopher Pfanstiel,
Syntegon Technology GmbH
Dr. Rainer Nicolai
F. Hoffmann – La Roche AG
Birger Bockius
Merck Electronics KGaA
Dr. Reinhold Maeck,
Boehringer-Ingelheim Corp. Center GmbH
Tim Luebke,
Bayer AG
Lukas Jürgen,
Boehringer-Ingelheim Corp. Center GmbH