SIG Pharma 4.0
About
In ISPE D/A/CH, members are actively involved in regional working groups and the CoP’s (Communities of Practice). Through technical/topic-based workshops, discussions, and communication, best practice working papers and approaches are developed in the sensitive environment of patient safety. They serve as guidelines for the industry and the authorities. Members of ISPE D/A/CH participate in the ISPE Guides.

Introduction
The ISPE Pharma 4.0TM Special Interest Group was formed in 2015 to develop forward-looking concepts on topics related to digitalization, Industry 4.0 and Smart Factory. The SIG Pharma 4.0TM works closely with regulatory authorities and stakeholders from the pharmaceutical industry.

The working groups
Since its foundation, the SIG has been working in six cross-functional working groups with the participation of members from the areas of Development, Quality, Manufacturing, Engineering, IT, Contracting, Technical Solution Providers, Consultants and Operators. All working groups are focused on fulfilling the common mission and establishing guidelines and best practices for the industrial transformation to Pharma 4.0.

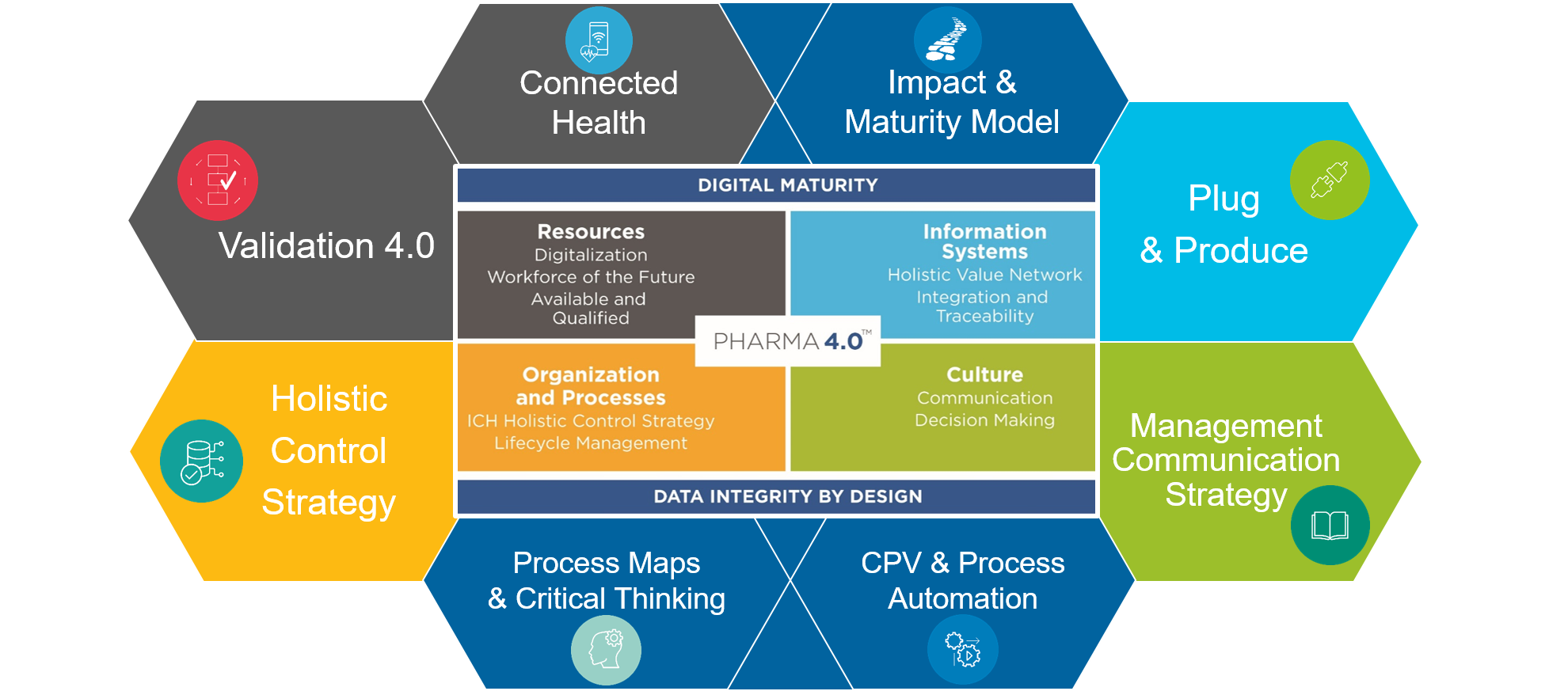
Want to learn more about the ISPE Pharma 4.0TM SIG? Read the story so far, successes and a vision for a digitized pharmaceutical industry in the Pharmaceutical Engineering Magazine >>>
The mission
The Pharma 4.0 Framework strategy is driven by the FDA vision of Dr. Janet Woodcock:
“A maximally efficient, agile, flexible pharmaceutical sector that reliably produces high quality products and services by a digitized, integrated & connected end to end supply chain.”
On it the group has adapted its mission:
“Manufacture pharmaceutical products with maximum product & process understanding, data integrity by design, efficiency and optimal resource allocation on the basis of full digital data transparency – to the benefit of the patient.”
Are you interested in active participation?
Network – Expertise – Progress
If you are interested in an active creative collaboration, please send an email to Christian Wölbeling.
Sample tasks for participation in all working groups: Writing technical articles, white papers, and good practice guides for practical application in industry, with regulatory acceptance.

Chair CoP - SIG Pharma 4.0 / Steering Committee
Christian Wölbeling
christian.woelbeling@ispe-dach.org
We would be pleased if you contact us directly.
Steering Committee
Christian Wölbeling
Werum IT Solutions GmbH
Chair